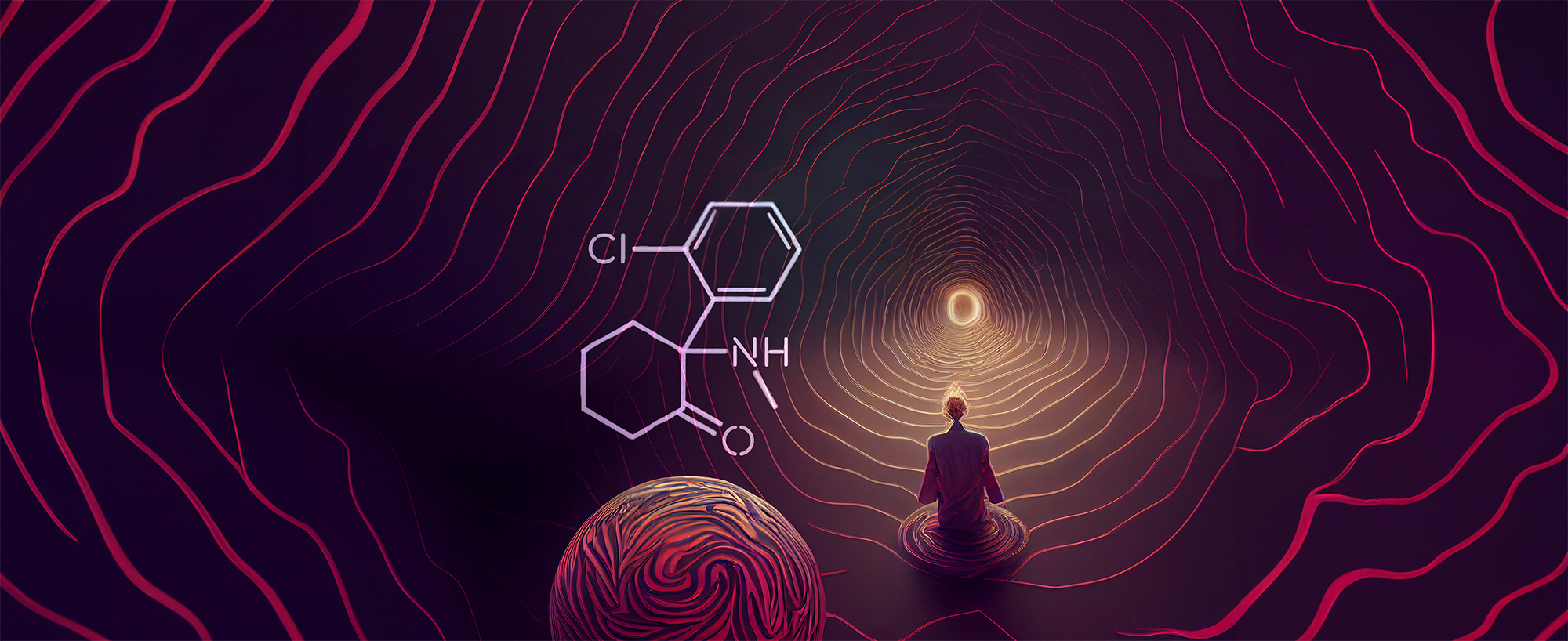
Lila Ash had long suffered from depression, and no treatment seemed to help. The few times she felt better, she had taken psychedelic drugs with friends. And yet, when her psychiatrist prescribed ketamine, she hesitated. She considered it a party drug, not a serious medicine. Eventually, though, she reached the point where she was willing to try it.1
After resting in a “calming room,” Lila was given noise-canceling headphones, an eye mask and a small intramuscular dose of ketamine.1 Five minutes later, she felt herself “disintegrating,” and all her fears and anxiety about ketamine faded away.1 Gone, too, were her persistent concerns about life. Sadness was replaced with feelings of deep love for her friends, her family and even herself.
When Lila returned to reality (about three hours later), she was no longer burdened with depression. “Because I hesitated to try ketamine,” she said, “I suffered longer than I had to. The treatment helped me look ahead with hope for my future”.1
For the past two decades, ketamine has been on the leading edge of research that is turning psychedelic drugs into clinically beneficial therapies for psychiatric disorders. And most amazingly, ketamine has proven effective in cases that are refractory to conventional therapy.
Too High for Comfort
In the 1950s, scientists at Parke-Davis & Company in Detroit were investigating a series of cyclohexylamines in their search for an “ideal” anesthetic drug with analgesic properties.2 On March 26, 1956, they synthesized phencyclidine, commonly called PCP.2,3
On September 11, 1958, Parke-Davis pharmacologist G. Chen received the compound for testing. Chen, along with Edward Domino, a pharmacologist at the University of Michigan, found that PCP was a potent analgesic in animals.2 But it also caused the appearance of drunkenness in rodents, delirium in dogs, cataleptic states in pigeons, and anesthesia in monkeys.3
When monkeys were given PCP, the researchers could perform surgery without eliciting pain, but the animals’ eyes were open, and their muscles had more tone—not less, which is typical of other anesthetics.2
Clinical trials were conducted at Wayne State University, and PCP was subsequently marketed as Sernyl®.2 It proved to be a safe and reliable clinical anesthetic.3 Just like in monkeys, PCP was also a potent painkiller and did not depress the patients’ cardiovascular or respiratory function.
Unfortunately, PCP’s usefulness was limited, because patients experienced postoperative excitation, delirium, and psychotic reactions, which sometimes lasted for hours.2,3 PCP was removed from the market in 1978. It was eventually classified as a Schedule I controlled substance (i.e., no accepted medical use and a high potential for abuse).
A Milder Shade of High
Despite PCP’s psychotic-like properties, Cal Bratton, head of pharmaceutical research at Parke-Davis, was unwilling to give up. He championed continued research, hoping to find an analog without PCP’s side effects and abuse potential.4 Calvin L. Stevens, a professor of organic chemistry at Wayne State University and a consultant to Parke-Davis, synthesized a series of PCP derivatives in his laboratory.3
In 1962, Stevens synthesized CI-581.2,3 Chen and his colleagues at Parke-Davis found that it produced excellent anesthesia in animals. Because CI-581 was a ketone with an amino group, they named it ketamine.2
Ketamine was less potent, considerably shorter acting, and most notably, caused less intense psychotic effects than PCP.2,3
Ketamine was less potent, considerably shorter acting, and most notably, caused less intense psychotic effects than PCP.
In early 1964, Parke-Davis asked Ed Domino to study ketamine in humans. Because Domino was not an anesthesiologist, he partnered with Guenter Corssen, a professor of anesthesiology at the University of Michigan who was studying intravenous anesthetics.2,3
On August 3, 1964, Domino and Corssen began their clinical trial, recruiting volunteers from the prisoner population at the Parnall Correctional Facility in Jackson, Michigan.2,3 Intravenous ketamine produced anesthesia and profound analgesia within 1–2 minutes. The effects lasted 5–10 minutes and could be safely prolonged with repeated infusions.5
The prisoners described vivid dreamlike experiences, the sensation of floating in outer space, and no feelings in their limbs. This altered state of consciousness as well as the post-surgical delirium were minimal compared to PCP. Respiratory depression was slight, and the prisoners exhibited modest increases in blood pressure and heart rate.5
As Domino and Corssen were writing their results for publication, they had long discussions about how to describe the novel ketamine effects on consciousness. They considered “schizophrenomimetic” and “dreaming”.2 Then, one evening, Domino described the effects to his wife, Toni, explaining that the men seemed “disconnected.” She suggested the term, “dissociative anesthetic.” Beginning with Domino and Corssen’s 1965 paper, ketamine (and subsequently other drugs in this class) has been described as a dissociative anesthetic.5
More recent electrophysiological and functional studies have found that “dissociative” drugs disrupt the connections between the brain’s thalamo-cortical and limbic systems.2
One evening, Domino described the effects [of ketamine] to his wife, Toni, explaining that the men seemed “disconnected.” She suggested the
term, “dissociative anesthetic.”
A Unique Anesthetic
Domino and his colleagues at the University of Michigan continued to study the pharmacology of ketamine.2 Its effects were quite distinct from all other anesthetics. Instead of depressing cardiovascular function, ketamine increased blood pressure, heart rate, and cardiac output.6 Ketamine caused only modest respiratory depression. Interestingly, it was also a potent bronchodilator with the unique ability to preserve upper airway reflexes. But it induced copious salivation, which in rare cases risked laryngospasm.3,6
Patients under ketamine-induced anesthesia were unresponsive to commands, but their eyes may have remained open. Their limbs moved involuntarily and, like with PCP, their muscle tone was increased.6
Parke-Davis patented ketamine in the US in 1966. It became available by prescription in 1969 under the trade name Ketalar® for veterinary use and was widely used for surgical anesthesia in animals.2,6 The Food and Drug Administration (FDA) approved ketamine (Ketalar) for human use in 1970.2,3
Ketamine is water and lipid soluble and can be safely administered intravenously, intramuscularly, subcutaneously, nasally, orally, and by other routes.3
Because of its rapid onset, short half-life, and impressive safety profile, ketamine became a popular induction anesthetic in a variety of patient populations and settings. Slow intravenous infusion minimized postoperative agitation.2,3
Ketamine was a popular battlefield anesthetic in Vietnam.2,3,7 Because it can be used safely in patients with elevated intracranial pressure (and may in fact be neuroprotective), ketamine was used in patients with traumatic brain injury.3,8 Its benefits included protection against seizures, cerebral ischemia, and secondary brain injury due to hypotension, and it continues to be used in military trauma medicine.3
Ketamine’s pharmacological properties and ease of use also made it a safe and effective anesthetic option in emergency field settings, such as mass casualty events.3,8
A Potent Painkiller
Ketamine provides a form of pain relief quantitatively and qualitatively similar to opioids.3,4 Unlike opioids, though, ketamine produces its analgesic effect while maintaining cardiopulmonary stability, airway reflexes, and respiratory function.2,3
Sub-anesthetic intravenous doses (0.2 to 0.8 mg/kg) produce good analgesia and sedation, and slow sub-anesthetic infusions (0.5 mg/kg per hour) produce continuous analgesia and sedation. Long-term infusion of a sub-anesthetic dose (10–40 mg per hour for 5 days) has been effective in relieving the pain of patients suffering from complex regional pain syndrome.4
Ketamine infusions may also provide an opiate-sparing alternative for managing post-operative pain and the pain of cancer patients who otherwise require high-dose opioids. It is also increasingly used as an adjunct for chronic pain. There are reports of efficacy in phantom limb pain, fibromyalgia, ischemic pain, and migraine with aura.3
Ketamine infusions may also provide an opiate-sparing alternative for managing post-operative pain and the pain of cancer patients who otherwise require high-dose opioids.
In burn patients, ketamine provides analgesia and sedation during debridement, grafts, and repeated dressing changes, without compromising the patient’s airway or respiratory function.3
Finally, the convenience of intramuscular administration, along with the wide safety margin, made ketamine one of the most commonly used drugs for sedation and analgesia in children, in emergency department patients, and in other uncooperative patients. Emergence excitability in children and teenagers is rare and typically mild.3
Efforts to explain the mechanisms underlying ketamine’s actions are providing researchers with new insights into the relationship between consciousness, anesthesia, and analgesia.3
Unlike most other anesthetics, which act primarily through potentiation of GABA transmission, ketamine has many molecular targets and neurophysiological properties.3
It reportedly interacts with NMDA, dopamine, serotonin, sigma, opioid, and cholinergic receptors, as well as hyperpolarization-activated cyclic nucleotide-gated channels.4
Its anesthetic effects are generally attributed to inhibition of N-methyl-D-aspartate (NMDA) receptors.4 There also appears to be a direct action on mu and delta opioid receptors (similar to morphine).4,9 Ketamine’s effect on pain seems to be mediated through both antagonism of NMDA receptors and activation of mu opioid receptors.4,9,10
Let’s Party
By the mid-1970s, ketamine gained additional popularity. Soldiers returning from Vietnam brought along their experiences with ketamine, leading to widespread recreational use in the U.S.2,4,11 In this wilder chapter of ketamine, sub-anesthetic shots became popular among psychedelic drug enthusiasts.10 Known on the street as “Special K,” ravers and other partiers were attracted to the swirly, out-of-body sensation that the drug produced.2,3,7,10–12
The “high” from a small intravenous infusion of ketamine was characterized by hallucinations and a distortion of time and space.3,4 Higher doses induced more severe, schizophrenia-like symptoms and perceptions that were completely separate from reality.3
Because of its potential for abuse, ketamine was classified as a Schedule III controlled substance in 1999 (i.e., moderate to low potential for physical and psychological dependence).
Testing at Yale
In parallel, a smattering of investigators suggested that ketamine might have antidepressant effects.2 The well-established standard-of-care for patients with major depression was a group of drugs that all targeted monoamine neurotransmitters. Those drugs were effective in many cases, but they caused a variety of unpleasant side effects. And sadly, about one-third of patients with major depressive disorder failed to respond.7,13–15
One alternative was electroconvulsive therapy, which was usually effective when antidepressant drugs failed, but not always.8,16
In the late 1990s, several lines of evidence suggested that dysfunction of the glutamatergic system might play an important role in depression.7,17,18 This prompted Dennis Charney and his team at Yale to explore whether ketamine (because it targets the glutamatergic NMDA receptor) possessed antidepressant properties.2,7
They enrolled seven patients who had not responded to standard antidepressants, a condition now generally called “treatment-resistant depression”.18 A sub-anesthetic dose of ketamine was infused intravenously. To Charney’s surprise, the patients felt better just hours after the infusion, and their depressant symptoms significantly decreased within 3 days after the single-dose treatment.18
The trial was double-blinded, but the investigators admitted that patients were able to discern between ketamine and placebo, because of the short-term psychedelic effect of ketamine.18 The Yale team published their results in 2000, but the medical community remained skeptical.7 It was a small trial, after all, and the difficulties maintaining double-blind conditions made the conclusions, at best, only suggestive of an effect.18
To Charney’s surprise, the patients felt better just hours after the [intravenous ketamine] infusion, and their depressant symptoms significantly decreased within 3 days after the single-dose treatment.
Replicating the Results
A few years later, Charney moved to the National Institutes of Health (NIH) and worked with a team headed by Carlos Zarate, Jr. They repeated the Yale study in 18 patients.7,17 All of the patients had been diagnosed with treatment-resistant depression, having tried an average of six antidepressant drugs without finding relief. A few had also failed to respond to electroconvulsive therapy.17
In a randomized, double-blind, placebo-controlled crossover study, the patients were given a 40-minute intravenous infusion of a sub-anesthetic dose of ketamine (0.5 mg/kg). Within 2 hours, the patients in the ketamine group showed significant improvement in their depression, compared to the placebo group, and the effect remained significant throughout the following week.17
Both the rapid onset and the long-lasting effect from a single ketamine dose were breakthrough findings. All of the known antidepressant drugs (MAO inhibitors, tricyclic antidepressants, and SSRIs) take 6–8 weeks to achieve their full effect. In the 2006 publication of the NIH results, Zarate said, “To our knowledge, there has never been a report of any other drug or somatic treatment…that results in such a dramatic, rapid, and prolonged response with a single administration”.17
Both the rapid onset and the long-lasting effect from a single ketamine dose were breakthrough findings.
It was an exciting finding. Ketamine was the first drug with a new mechanism of action to treat depression in more than half a century.7
Following the NIH report, other researchers confirmed Zarate and Charney’s results.7,11 Off-label use of intravenous ketamine for treatment-resistant depression became widespread. Patients who had gone through many antidepressant drugs (as well as talk therapy and even electroconvulsive therapy) without success were very willing to try other treatment options.8,11,19 Ketamine often met that need.
Anesthesiologists and primary care physicians, along with some academic medical centers, accounted for most of the off-label use.19 But increasingly, entrepreneurial practitioners set up freestanding specialty clinics to offer off-label ketamine (and perhaps other psychedelic drugs) to patients with depression.12
For some patients, ketamine was life-changing. Others did not respond.7,12 There were no standard guidelines.8 In most cases, intravenous ketamine seemed to relieve depressive symptoms after 1–3 treatments. In patients who experienced relief, subsequent sessions prolonged the antidepressant effect but did not produce any further dramatic relief of symptoms. Many practitioners offered eight treatments initially, and then the patient and doctor decided whether to taper or stop ketamine treatments, or to continue at longer dosing intervals.8
Proposed Mechanisms
The rapid antidepressant effect of ketamine has been attributed to antagonism of NMDA receptors. But this relationship is not completely understood, because other NMDA antagonists have failed to elicit the same robust effect.7,11
Ketamine is a non-competitive NMDA receptor antagonist and causes an increase in glutamate, the main excitatory neurotransmitter in the brain.4,7,8,14,20 Specifically, ketamine binds to NMDA receptors on GABAergic inhibitory interneurons.3,11 Inhibition of those interneurons leads to a sudden increase in glutamate, called the glutamate burst, which is apparently responsible for the rapid effect of ketamine on depression.7,11,21
The elimination half-life of ketamine is 3–4 hours, and for a long time, researchers were puzzled by the drug’s extended clinical efficacy, which typically lasted for about 2 weeks after a single dose.6,17,21 The explanation seems to be rapid restoration of disrupted synaptic connections in the brain.
The glutamate system is very important in learning, memory, and other brain functions. In depression, glutamate synapses and circuits appear to be dysregulated.11 Studies of chronic stress in rodent models, as well as clinical depression in patients, have revealed that neurons wither, and many synapses are lost.13 Decreased synapse density has also been observed by electron microscopy in the prefrontal cortex of patients with depression.13
The burst of glutamate induced by ketamine stimulates downstream brain pathways, leading to protein synthesis and rapid increases in the number of synapses and spine density, particularly in the prefrontal cortex and hippocampus.7,13,21 The repaired synapses and brain circuitry help to restore brain homeostasis.11,13,15
Researchers now think that the direct action on glutamate accounts for ketamine’s rapid effect, whereas the older monoamine antidepressants act on these pathways more indirectly. And they think ketamine’s glutamate-triggered restoration of brain synapses is key to its long-lasting antidepressant effect.7
The Downside
Although ketamine proved to be less hallucinogenic than PCP, psychoactive effects still limit its clinical use.3
A single sub-anesthetic intravenous dose commonly produces dissociation and both positive and negative psychotic-like effects. The dissociation effects include distortions in visual, auditory, or somatosensory stimuli, and alterations in the perception of self or time. The positive psychotic-like effects include conceptual disorganization, hallucinations, suspiciousness, and unusual thought content. The negative psychotic-like effects include blunted affect, emotional withdrawal, and motor retardation.17 These effects are dose-dependent and subside within 40 minutes after treatment termination. Repeated sub-anesthetic doses, over time, have been shown to lessen these effects.17
Ketamine’s other side effects are also dose-dependent and self-resolving. Cardiopulmonary toxicity is rare and due to transient sympathetic-induced increases in heart rate and blood pressure.3
A Suspected Fatality
There is no known lethal dose of ketamine in humans.4 Death from overdose is rare and usually involves other intoxicants or an accompanying trauma.3,4 Ketamine’s effects can be compounded when it is combined with other drugs that alter mood and perception, such as alcohol, opioids, benzodiazepines, and cannabis. Accidental deaths from falls, extreme hypothermia, and car crashes involving ketamine have also been reported.4
There is no known lethal dose of ketamine in humans. Death from overdose is rare and usually involves other intoxicants or an accompanying trauma.
In October 2023, actor Matthew Perry was found floating face-down in his hot tub.22 Later, the Los Angeles Police Department, Drug Enforcement Agency, and U.S. Postal Service opened an investigation into Perry’s death, because the medical examiner found an anesthetic level of ketamine in his blood.22
Perry had been undergoing ketamine infusion therapy every other day but had recently reduced the dosing frequency. His last known infusion was 10 days before his death. Perry was also taking the opioid, buprenorphine, to treat his opioid use disorder. No other drugs or alcohol were in his system.22
As complicating factors, Perry had diabetes, coronary artery disease, and chronic obstructive pulmonary disease. Until recently, he had been a two-pack-a-day cigarette smoker.22
Perry’s high blood level of ketamine was puzzling, because ketamine (with its short half-life) should have cleared his system within hours after his last documented dose. Consequently, the focus of the officials’ investigation was to determine the source (presumably illicit) of the ketamine in his system and how it got there. They were able to link several people to procurement of the drug.22
The high blood level of ketamine, although not fatal on its own, could have induced delusions and loss of motor control. In addition, ketamine’s anesthetic effect could have been enhanced by Perry’s opioid intake, poor pulmonary function, and cardiovascular disease, leading to accidental drowning.22
On August 15, 2024, five people, including Perry’s live-in assistant, were charged with selling or administering the ketamine that led to his death.
Moving the Needle
The robust and now widely acknowledged antidepressant efficacy of ketamine prompted more formal acceptance—and regulation—of its use in treatment-resistant depression.
In 2017, an American Psychiatric Association task force said there was “compelling evidence” to support the antidepressant effects of ketamine infusion, despite gaps in the clinic al data.11 The task force’s chief concerns were ketamine’s long-term safety and durability, which had not yet been adequately studied. That data would be needed to properly guide its use.
Ketamine is a racemic mixture of the S(+) isomer, known as esketamine, and the R(-) isomer, known as arketamine. Esketamine has 3- to 4-fold higher binding affinity to NMDA receptors than arketamine.14 It is about 3-times more potent for anesthesia and analgesia than arketamine.4,11 Esketamine is also associated with less cardiac stimulation, less spontaneous motor activity, fewer psychotic-like side effects, lower incidence of emergence delirium, and more rapid recovery than arketamine.3
Making it Official
In 2016, researchers at Janssen Pharmaceuticals published a report of esketamine’s effect on treatment-resistant depression.14 Thirty patients were given a slow intravenous infusion of sub-anesthetic doses of esketamine in a randomized, double-blind, placebo-controlled study. Like ketamine, esketamine produced a rapid, robust, and persistent improvement in the patients’ depressive symptoms after a single dose.14
This was the first study to profile esketamine in treatment-resistant depression, and the isomer’s efficacy was both statistically significant and clinically meaningful.14
Janssen decided to move forward with development of a commercial esketamine product in the form of a nasal spray. The nasal spray would be absorbed about as rapidly as intravenous infusion but would be easier for doctors and patients to administer.7 Because of the anticipated clinical advantages of esketamine compared to conventional antidepressant drugs, the FDA designated the compound as a Breakthrough Therapy, which facilitated its rapid clinical development.15
In randomized double-blind clinical trials (TRANSFORM), patients with treatment-resistant depression were given an oral antidepressant drug, along with either esketamine or placebo.15,16 The benefits of esketamine were apparent within 1 day, and the effect remained statistically significant for 28 days.15
This was the first time that an antidepressant (esketamine) was shown to be superior to an active comparator (an oral antidepressant drug) in any clinical trial of major depressive disorder.15
In follow-up studies (SUSTAIN), continued treatment of the patients with twice-a-week dosing for 18 months showed that relapse of depressive symptoms was significantly less likely to occur in the esketamine-treated group than those receiving placebo.16
On March 5, 2019, the FDA approved esketamine nasal spray (Spravato®) for use in combination with an oral antidepressant for treatment-resistant depression.23 Janssen’s product was the first FDA-approved esketamine for any use. However, because of the risk of sedation and “dissociative” effects, and the potential for abuse and misuse, Spravato was classified as a Schedule III controlled substance, and its use was further restricted by a Risk Evaluation and Mitigation Strategy (REMS) program.23
Under REMS, pharmacies and healthcare personnel who purchase, dispense, and/or supervise administration of esketamine must be FDA-certified.16,23 Only patients enrolled in the REMS program may receive the drug, and they must be monitored during and for at least two hours after drug administration. Esketamine cannot be dispensed directly to the patient for at-home use.16,23
This was the first time that an antidepressant (esketamine) was shown to be superior to an active comparator (an oral antidepressant drug) in any clinical trial of major depressive disorder.
Addressing Suicide, Finally
Depressed patients with suicidal ideation show more severe symptoms and respond less well to treatment, compared to those without suicidal thoughts.24 In fact, clinical trials of antidepressant drugs have excluded patients exhibiting suicidal behavior, because such patients were considered the least likely to respond.24–26
The prevalence of suicidal ideation in major depressive disorder is as high as 60%, and the lifetime incidence of attempted suicide in this population is 10–20%.24,26 These patients constitute a psychiatric emergency, and until recently, there were no approved emergency medications. The standard of care included initiation or optimization of oral antidepressants, and frequently, patients were hospitalized to prevent self-harm. The benefits of hospitalization were short-lived, and the risk of attempted and completed suicide remained high in the weeks immediately following discharge.24,26
The time between onset of suicidal thoughts and suicide attempt is often very short, highlighting the need for immediate intervention.8,17,26 Unfortunately, the 6- to 8-week lag in onset of conventional antidepressant drugs limits their utility in these crisis situations.
Because of ketamine’s fast-acting antidepressant effect, it was reasonable to suppose that it might also benefit those at high risk of suicidal behavior. In addition to esketamine’s demonstrated efficacy in treatment-resistant depression, Janssen’s clinical trials provided evidence that esketamine might also decrease suicidal thinking.16,26
Again, the FDA designated esketamine nasal spray as a Breakthrough Therapy for major depressive disorder with imminent risk for suicide. That facilitated the drug’s development for this new indication.15
Janssen’s clinical trials (ASPIRE I and ASPIRE II) marked a major milestone in investigations of antidepressant drugs. Esketamine nasal spray (Spravato), in combination with an oral antidepressant, reduced the severity of patients’ suicidality within 24 hours.24–26 The benefit was greater in the esketamine plus oral antidepressant group than those receiving only a standard-of-care antidepressant, and the esketamine effect lasted up to four weeks.24–26
On August 3, 2020, the FDA approved esketamine nasal spray for treatment of patients with major depressive disorder and acute suicidal ideation or behavior.25 It was the first drug approved for this indication and may fulfill the unmet need for a rapidly acting drug in this patient population.24,25
Off-label Popularity
Since the approval of ketamine in 1970, physicians have been legally permitted to prescribe it for any medical condition. Consequently, after the discovery in the early 2000s that sub-anesthetic doses rapidly diminished the symptoms of major depression, practitioners began administering it off-label.3,10
Although many of these healthcare providers carefully screen their patients and diligently monitor them during and after treatment to manage any hallucinatory side effects, the entrepreneurial practitioners at specialty/standalone clinics do not comply with the strict regulatory requirements imposed on Spravato. Clinics, like the one where Lila received her treatment, administer ketamine (the racemate) by intramuscular injection, not esketamine by nasal spray. And they do not administer ketamine in combination with an optimized regimen of an oral antidepressant, which is required for Spravato administration.
In addition, they do not follow the procedures for drug storage, accountability, and reporting that are required by REMS for Spravato. This is especially concerning, because there is little data on the effects of long-term, low-dose administration of ketamine/esketamine.16,25 Similarly, controlled clinical trials of ketamine’s abuse potential are lacking.4
Hints of Concern
Long-term studies are needed both to determine the durability of the antidepressant effect and to establish the safety of long-term exposure.4,7,8,19 Toxicology testing in animals suggests that long-term administration may cause long-lasting changes in brain neuronal circuitry and perhaps irreversible changes in behavior.4
Despite the lack of controlled trials, the effects of chronic exposure have been derived from recreational use.4 In a one-year observational study of recreational users, frequent use (more than four-times per week) was associated with impaired short-term and long-term memory.14 But those memory-related deficits have been difficult to link directly to ketamine, because of complicating comorbid and environmental factors.4
Long-term use may also lead to flashbacks, attentional dysfunction, and decreased sociability. There may also be pronounced and persistent neuropsychiatric effects, including schizophrenia-like symptoms, cognitive impairment, and poor psychological well-being.3,4
Despite its abuse potential, instances of ketamine dependence are relatively rare, although some isolated cases have been reported. There is also evidence suggesting that repeated ketamine use may lead to drug tolerance.4
More to Come
The antidepressant efficacy of ketamine has inspired researchers to explore it for other mental and emotional disorders.3,7,10,11 Clinicaltrials.gov lists dozens of clinical trials investigating ketamine for PTSD, obsessive-compulsive disorder, bipolar disorder, and anxiety, among others. Researchers are also conducting structure-activity studies to try to maximize ketamine’s antidepressant efficacy and minimize its psychotic-like properties.
Ketamine’s impressive track record has also sparked interest in other psychedelic drugs including psilocybin, LSD, ayahuasca, and MDMA (ecstasy). Those compounds are also being studied for treatment of depression, PTSD, obsessive-compulsive disorder, bipolar disorder, and anxiety.3,7,11,20 But that’s another story.
Author
-

Rebecca J. Anderson holds a bachelor’s in chemistry from Coe College and earned her doctorate in pharmacology from Georgetown University. She has 25 years of experience in pharmaceutical research and development and now works as a technical writer. Her most recent book is Nevirapine and the Quest to End Pediatric AIDS.
View all posts